Taking Care of Your Robots Power Source
Index:
Introduction
Ni-Cd
Charging
Discharging
Safety
NiMh
Charging
Discharging
Safety
Sealed Lead Acid
Charging
Discharging
Safety
Li-ion
Charging
Discharging
Safety
Disclaimer
Bibliography
Introduction
One of the single most expensive components that go to make up an amateur mobile robot is its power source. Of course, I'm speaking of the batteries that supply the current necessary to power motors, sensors and processors on board the robot (unless any of you have worked the bugs out of cold fusion, we'll assume you're using batteries!).
The goal of this article is to give you the information necessary to take good care of your batteries and to do it safely. Issues such as correct charging parameters, discharging, shelf life and cycle life will be explored. Rechargeable battery technology (excluding the so-called "renewable" alkaline) such as sealed lead acid, Nickel-Cadmium, Nickel Metal Hydride and Lithium-ion will be considered.
Ni-Cd (nickel-cadmium)
Let's start with Ni-Cd chemistry because this is probably the most popular battery out there. When properly charged and discharged, a Ni-Cd battery can maintain its capacity through 500 charge cycles, and last more than 5 years. While Ni-Cds will discharge if left "on the shelf", they can be renewed to near 100% of capacity after a few charge cycles. However, if you're working in really cold weather, you may want to go to another type battery because while charging at temperatures below 0° C, the gas absorption reaction is not adequate and the battery may be damaged.
Charging
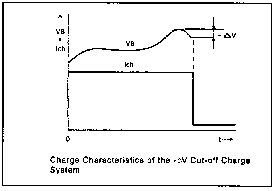
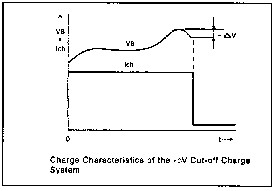
One great thing about Ni-Cds is that they can be charged very rapidly without damaging them--provided the charge process is carefully controlled to avoid overcharging and the battery pack is well ventilated to avoid overheating. A popular charge method known as -DV (see the chart below) is used to charge cells in as little as one hour.
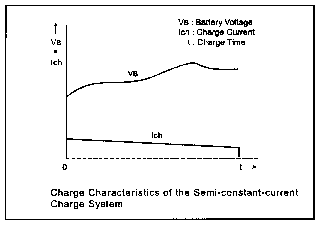
The simplest charging method is known as the "semi-constant current charge".
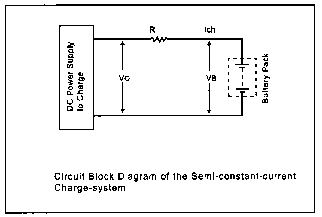
In this scheme, a series resistor is used in the charge path to stabilize the charge current. And the battery is simply charged for about 15 hours. Single cell battery voltage, VC is set to 1.45 volts, so your battery voltage is determined by (VB = VC x N), where N is the number of cells used. Using this method, the battery will be charged at a 0.1 CmA rate. Charge current (Ich ) is given by:
Ich = Vo - Vb / R
Let's work through an example. If you are working with a 6 cell battery pack composed of AA cells that are rated at 1,000 mAH, the parameters will be calculated as follows:
Vb = 1.45 x 6 or 8.7V. This is our fully charged state.
We will want to charge the battery at a 0.1 C rate. Since our batteries are rated at 1,000 mAH, we will be supplying 100 mA of current to the battery during the charge cycle. Our resistor will be 8.7V- 7.2V/0.1A = 15 ohms. The 7.2V is derived from multiplying the rated cell voltage of 1.2 volts times the number of cells in the battery pack (6).
The idea is that by the time the cells are fully charged, the current will drop to nearly zero. Using this method will help prevent overcharging and over heating of your battery pack, thus preventing damage. Be sure to choose a resistor large enough to handle the power needed. Semi-constant current charging is a safe, inexpensive way to charge your NiCads.
Discharging
Design your system to discharge the battery pack at a 0.1 to 0.5 C rate for highest efficiency. Overheating your battery at high discharge rates will lead to deterioration in battery performance. In any case, it's not a good idea to discharge your battery at more than 2 C rates for any extended period of time. Over discharging WILL damage your battery pack.
Use the table below to determine minimum voltage on your cells.
Number of arranged serially batteries
1 to 6
(Number of batteries x 1.0) V
7 to 20
((Number of batteries -1) x 1.2) V
The point here is to make sure you have a way to monitor battery voltage in your application and cut the power when minimum discharge levels are reached.
Safety
Very simple and straight forward rules that must be mentioned here:
1. Don't disassemble the individual cells.
2. Never short-circuit these cells.
3. Observe polarity when charging and discharging...don't intentionally reverse polarity on these batteries.
4. Recycle nickel-cadmium batteries, don't throw them in the landfill. Cadmium is a nasty contaminant.
5. Don't attempt to solder directly to the case of the cell. You could damage the safety vent or otherwise mess-up the battery.
NiMH (Nickel-Metal-Hydride)
This battery chemistry is a little harder to find and even more expensive than Ni-Cd batteries. The attractive feature of this battery is its high current density. Generally, NiMH batteries of equivalent size and weight to Ni-Cd will have higher amp-hour ratings-up to 2X higher. They are similar to NiCads in that they have the same per-cell nominal voltage of 1.2 V and their charge and discharge curves are the same.
One important difference is that NiMH batteries cannot be discharged at real high rates because they tend to generate more heat in the discharge process and this could lead to loss of electrolyte and damage to the battery. Keep the continuous discharge rate below 2C. Also, like NiCads, these batteries will develop a "memory" if they are continously cutoff before discharging below 1.1 V/cell, in turn having an effect on capacity. This can be remedied by a few cycles of proper charging and discharging. Cycle life and shelf life of this battery is similar to NiCad.
Charging
Nickel-metal batteries should not be trickle charged for extended periods of time. They will likely overheat and damage will occur through venting of electrolyte. The best way to charge these batteries is designing a "smart" charger that will cutoff when one of several conditions have been reached (see chart below). If your battery pack is deeply discharged (about 0.8V per cell) the pack must be trickle charged up to about 1.1V before rapid charging takes place.
Charging algorithms include such schemes as charge cutoff pegged to the rate of temperature rise (6), charge terminating voltage of 1.8 V (4), sensing the -DV droop at the end of the charge cycle (5) and so on.
I have found that having a constant current power supply, setting the current limit to 1C and keeping an eye on voltage and current over time do the trick quite well, but one must be alert and not leave the battery on the supply for long periods of time!
Discharging
All of the same rules stated above for the nickel-cadmium technology apply to NiMH cells. The only exception is that discharging these cells at real high rates (>3C) will more than likely damage them.
These batteries are most efficient while discharging between 0.1C and 0.5C.
For determining the discharge end voltage, see the table above. It is the same as that of NiCad technology.
Safety
Again, there is not much to add to what has been said for the Ni-Cd type batteries. All rules that apply there should apply here. Most OEM battery packs have 3 or 4 terminals: battery positive and negative and thermistor. NiMH batteries tend to generate more heat when rapidly charging and discharging than other types of cells, so manufacturers include a thermis-tor within the battery pack to monitor temperature.
Sealed Lead Acid (SLA)
Over the past decade, sealed lead-acid batteries have become widely available to the robot experimenter. Most of these batteries are of the 6 and 12 volt varieties, with a wide range of Amp-Hour ratings. If you need loads of current at relatively low prices and aren't concerned with weight, this is the battery for you. Sealed lead-acid batteries have great advantage over their flooded-cell cousins because the electrolyte is sealed up and they don't produce prolific amounts of hydrogen gas as they charge. Everyone has heard stories about car batteries exploding because a spark ignited the hydrogen gas emitted by these things.
Because sealed lead acid batteries are used in a wide variety of applications, there are a number of maintenance schemes designed to meet the needs of each application. Mobile robots will cycle their batteries frequently as opposed to a long-term storage application. Therefore, charging parameters will be based on relatively rapid charge cycles rather than "float" or "trickle" charging.
Charging
Nominal voltage on SLAs is 1.75 volts per cell under loaded conditions and cells can be charged to as high to 2.45 volts. Probably the most popular charging method for this chemistry is the "constant voltage" method.
Click for bigger pic
Simply apply a voltage of 2.45 V / cell and allow the battery to charge to this value. There are 6 cells that make up a 12V battery so charge voltage would be set to 14.7Volts. You must assure the power source is adequate to supply the current the battery demands or that the power supply can "crowbar" to protect its circuits using this method. Manufacturers recommend that current should be limited to 0.4C at the beginning of the charge cycle. Charge time will generally be less than 3 hours depending on the state of discharge.
Discharging
One key feature of maintaining long battery life with an SLA battery is watching the "depth of discharge" of your battery as it goes about it's daily life. If you design your application to discharge no more than 30% of capacity, the battery can easily be "cycled" a thousand times! Conversely, if you want to wear your battery out fast, deep discharge it (50%) often and you will quickly be sending it to the recycler.
Ok, having said that, it is still worthy of note that unlike their NiCad and Nickel-metal brethren, lead acid batteries can be deeply discharged and still come back for more. Doing this just reduces the number of charge cycles you can get out of the battery.
As with other battery chemistries, storing lead-acids will allow them to self-discharge over time. This is no problem if they are not left on the shelf too long. A few charge-discharge cycles will bring the battery back up to full capacity. However, if allowed to remain on the shelf uncharged for too long, sulfation of the plates can occur leading to reduced capacity. Batteries in storage should be recharged at least annually, depending on storage temperature.
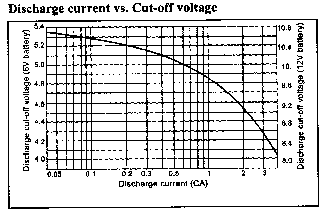
The state of discharge can be determined by measuring the open circuit voltage of the battery. The graph below shows the residual capacity vs. the open circuit voltage of the battery.
Safety
All of the points raised in the section on NiCad batteries should be observed here. Additionally, while sealed lead-acid cells don't release a lot of hydrogen gas when charging, they do release small amounts depending on temperature and charge rates. Therefore, do not use this battery in tightly enclosed applications where gases can collect. Finally, DO recycle that dead lead-acid battery.
Lithium-Ion (Li-Ion)
These are the Ferraris of the battery world.
Because we are seeing more of this chemistry in portable products today, it is worth discussing this technology since more and more experimenters will be getting their hands on it. These batteries are the ultimate in light weight, high power density, high per-cell voltage and high cost. This battery also has excellent shelf life.
At the same time, use of Li-Ion batteries comes at a rather high risk. If not handled properly, these cells can ignite or explode. Every cell has several protection features built in. Each cell contains:
1. Special vents built into the battery case.
2. A small circuit board complete with micro-controller to monitor charge and discharge conditions and open the circuit if things go awry.
3. A fuse
4. A built-in thermistor.
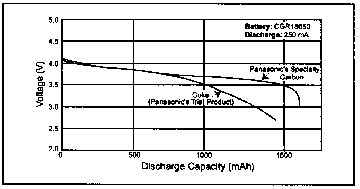
As mentioned earlier, the power density of this battery is awesome. The chart below illustrates this fact. Part of what makes the power density so great is the fact that each Li-Ion cell produces 3.7V under load, equivalent to 3 Ni-Cds. Moreover, lithium batteries have none of the "memory" effects seen in Ni-Cd and NiMH batteries. Lithium batteries exhibit an extremely flat discharge voltage profile throughout the discharge period.
Aside from the risky character of this chemistry, its other limiting factor is that the cells max-out at about 1,400 mAH capacity. No doubt manufacturers will continue to increase this number, but for now, that's it. I haven't had the opportunity to read much about the newest Lithium technology-lithium polymer. By using the polymer as an electrolyte, the batteries are expected to be much less volatile and can be shaped to most any form. This technology is just hitting the OEM market as of this writing.
Charging
While there are a half-dozen charging schemes for the other chemistries, there is only one for Li-ion...the carefully controlled "constant voltage/constant current" method. Here are the recom-mended parameters to keep in mind as the battery is charged. First, the maximum voltage of the cell is 4.2V. If charging is cutoff at 4.1V, the battery will be about 80% charged. Do not exceed 4.2V. Second, charge at a rate not to exceed 1C. Panasonic recommends a 0.7C rate. When cell voltage is 2.9V or less, begin the charge at a 0.1C rate. Third, charge at temperatures between 0°C and 45°C. The on-board controller will not allow charging to more than 4.3 volts.
Discharging
Current should be maintained at 1.0C or less. If discharging a 1,000mAH battery, limit the current to a maximum of 1,000mA. Most likely the on-board controller will limit the amount of current that can be drawn from the cell as a form of short-circuit protection. You might find that under loads greater than 1C, even for very short periods of time, your battery will suddenly "go dead" when the controller kicks in. Terminate discharge of the cell at 3.0 volts. Below 2.7V the cell will likely suffer damage. The on-board controller will probably not allow discharge to this level.
As can be seen from the graph above, the discharge voltage remains flat and rolls off steeply at the end of the cycle. Note cutoff at 3 volts.
Safety
I have heard some remarkable stories about Lithium-Ion batteries and their volatile behavior. Plants producing these batteries in Japan have burned twice over the past few years. Cellular phones are rumored to have ignited while the user was in a phone call. There is a story of a lithium-ion battery in an Apple laptop igniting at a press conference announcing the new computer. In other words, these batteries have attained legendary status in their short history. Please be very careful with these devices. Here's the list:
1. Do not reverse polarity while charging or discharging these cells!
2. Do not allow the cell to receive a sharp or sudden impact!
3. Do not place the battery in fire or heat the battery!
4. Do not carry or store the batteries together with necklaces, hairpins, or other metal objects.
5. Do not pierce the battery with nails, strike the battery with a hammer, step on the battery or otherwise subject it to strong impacts or shocks! See number 2 above.
6. Do not solder directly to the battery!
7. Do not expose the battery to water or salt water, or allow the battery to get wet.
8. Do not modify or remove the safety and protection devices!
9. Do not place the batteries into direct sunlight.
10. Do not leave the battery in a car on hot days!
This list was quoted from the Panasonic "Safety Precautions for Lithium Ion Battery Packs".
Taking good care of your robot's batteries will assure maximum return on your investment. Please check out the bibliography for more information on the subject of batteries.
Standard Disclaimer
This paper is designed to help the robot hobbyist gain some understanding for the care and feeding of batteries. It is for educational purposes only. Please consult manufacturer's data sheets in order to design your battery application. The author will not be held responsible for accidents or injuries resulting from use of the information herein.
Greg Preston
Triangle Amateur Robotics Club
GoRobotics.net - used with permission
August 7, 2000
Bibliography:
Panasonic OEM Battery web pages
Batteries in Your Shack
Raleigh Amateur Radio Society Newsletter, June-August, 2000
Jeff Wittich
Thanks for helping to keep our community civil!
This post is an advertisement, or vandalism. It is not useful or relevant to the current topic.
You flagged this as spam. Undo flag.Flag Post